State whether the following statement is true or false. If false, rewrite the correct form of statements.
The K-shell of an atom can hold a maximum of electrons.

Important Questions on Atomic Structure
State whether the following statement is true or false. If false, rewrite the correct form of statements.
The nucleus of an atom is positively charged.
State whether the following statement is true or false. If false, rewrite the correct form of statements.
J J Thomson discovered the neutrons.
State whether the following statement is true or false. If false, rewrite the correct form of statements.
The atomic number of calcium is . So, its electronic configuration is .
State whether the following statement is true or false. If false, rewrite the correct form of statements.
A chloride ion is positively charged.
Look at the diagram given below and answer the following questions:
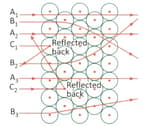
Name the particles used by Rutherford, for this experiment.
Look at the diagram given below and answer the following questions:
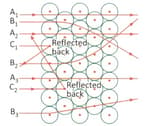
What is the nature of charge on these particles, positive or negative?
