MEDIUM
JEE Main
IMPORTANT
Earn 100
Statement - I : When amount of an ideal gas undergoes adiabatic change from state to state , then work done is , where and universal gas constant.
Statement - II : In the above case, when work is done on the gas, the temperature of the gas would rise.
83.72% studentsanswered this correctly
Important Questions on Thermodynamics
EASY
JEE Main
IMPORTANT
The total internal energy of two mole monoatomic ideal gas at temperature will be _____ . (Given )
MEDIUM
JEE Main
IMPORTANT
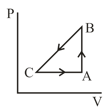
A sample of an ideal gas is taken through the cyclic process as shown in figure. It absorbs, of heat during the part , no heat during and rejects of heat during . A work of is done on the gas during the part . The internal energy of the gas at is . The work done by the gas during the part is
MEDIUM
JEE Main
IMPORTANT
A cylinder of fixed capacity of litres contains helium gas at standard temperature and pressure. The amount of heat needed to raise the temperature of gas in the cylinder by will be (Given gas constant )
EASY
JEE Main
IMPORTANT
of heat is given to a heat engine, and it rejects of heat. If source temperature is , then the temperature of sink will be _____
MEDIUM
JEE Main
IMPORTANT
Starting with the same initial conditions, an ideal gas expands from volume to in three different ways. The work done by the gas is if the process is purely isothermal, , if the process is purely adiabatic and if the process is purely isobaric. Then, choose the correct option
EASY
JEE Main
IMPORTANT
Let is the efficiency of an engine at and while is the efficiency at and . The ratio will be
EASY
JEE Main
IMPORTANT
mole of certain monoatomic ideal gas undergoes a temperature increase of at constant pressure. The increase in the internal energy of the gas in this process is
(Given )
MEDIUM
JEE Main
IMPORTANT
A monoatomic gas at pressure and volume is suddenly compressed to one eighth of its original volume. The final pressure at constant entropy will be
