Statement I: For colloidal particles, the values of colligative properties are of small order as compared to values shown by true solutions at same concentration.
Statement II: For colloidal particles, the potential difference between the fixed layer and the diffused layer of same charges is called the electrokinetic potential or zeta potential.
In the light of the above statements, chooses the correct answer from the options given below.

Important Questions on Surface Chemistry
The number of statement/s which are the characteristics of physisorption is ___________.
A. It is highly specific in nature
B. Enthalpy of adsorption is high
C. It decreases with increase in temperature
D. It results into unimolecular layer
E. No activation energy is needed
The variation of the rate of an enzyme catalyzed reaction with substrate concentration is correctly represented by graph
(a)
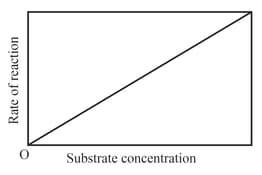
(b)
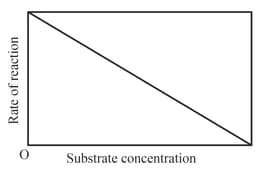
(c)
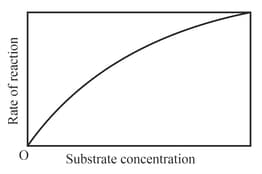
(d)
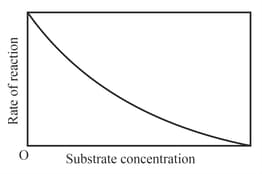
The number of incorrect statement/s from the following is/are
A. Water vapours are adsorbed by anhydrous calcium chloride.
B. There is a decrease in surface energy during adsorption.
C. As the adsorption proceeds, becomes more and more negative.
D. Adsorption is accompanied by decrease in entropy of the system.
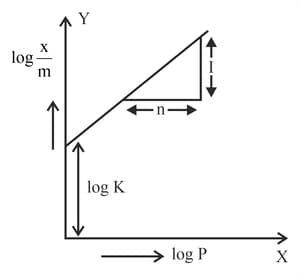
The graph of for an adsorption process is a straight line inclined at an angle of with intercept equal to . The mass of gas adsorbed per unit mass of adsorbent at the pressure of is _____ (Nearest integer)
Given :
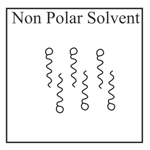
Adding surfactants in non polar solvent, the micelles structure will look like
(a)
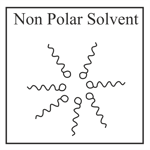
(b)
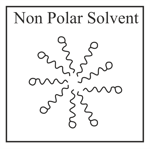
(c)
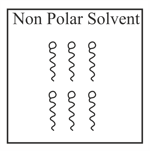
(d)
Match List I with List II
| List I | List II | ||
| A | Physisorption | I | Single Layer Adsorption |
| B | Chemisorption | II | |
| C | III | Chromatography | |
| D | Analytical Application or Adsorption | IV | Heterogeneous catalysis |
Choose the correct answer from the options given below:
In figure, a straight line is given for Freundrich Adsorption . The value of and are respectively.