HARD
Earn 100
Statement-I. molecule is more stable than molecule.
Statement-2. The antibonding electron in the molecule destabilises it.
(a)Statement-I is True, Statement-2 is True; Statement-2 is a correct explanation for Statement-I
(b)Statement-I is True, Statement-2 is True; Statement-2 is not a correct explanation for Statement-I.
(c)Statement-I is True, Statement-2 is False.
(d)Statement-I is False, Statement-2 is True.
50% studentsanswered this correctly
Important Questions on Chemical Bonding and Molecular Structure
EASY
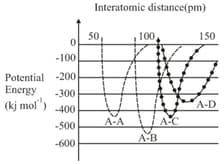
EASY
Assertion: In the bonding molecular orbital (MO) of electron density is increased between the nuclei.
Reason: The bonding MO is , which shows destructive interference of the combining electron waves.
MEDIUM
EASY
How many ions of the following have a bond order of
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
EASY
EASY
EASY
MEDIUM
The bond lengths (in of and are:
MEDIUM
MEDIUM
MEDIUM
MEDIUM
HARD
MEDIUM
HARD
Decreasing order of stability of and is:

