HARD
Earn 100
Statement-I. The boiling point of lies between that of and .
Statement-2. has much lower boiling point than but it increases from to to to due to increase in van der Waals forces.
(a)Statement-I is True, Statement-2 is True; Statement-2 is a correct explanation for Statement-I
(b)Statement-I is True, Statement-2 is True; Statement-2 is not a correct explanation for Statement-I.
(c)Statement-I is True, Statement-2 is False.
(d)Statement-I is False, Statement-2 is True.
50% studentsanswered this correctly
Important Questions on States of Matter
EASY
Increasing order of boiling points in the following compounds is:
EASY
MEDIUM
Match the type of interaction in column with the distance dependence of their interaction energy in column
| A | B |
| (i) ion - ion | (a) |
| (ii) Dipole - dipole | (b) |
| (iii) London dispersion | (c) |
| (d) |
EASY
[ is the distance between the polar molecules]
MEDIUM
EASY
EASY
HARD
EASY
EASY
MEDIUM
In which of the following solid substance dispersion forces exist?
MEDIUM
Find out the correct order of ionic character in the following molecules
MEDIUM
Given:
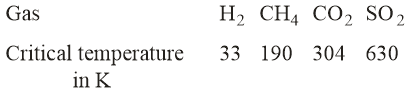
On the basis of data given above, predict which of the following gases shows the least adsorption on a definite amount of charcoal?
MEDIUM
MEDIUM
EASY
(Latent heat of ice is and )
MEDIUM
EASY
EASY
EASY

