Strips of copper foil and magnesium ribbon were cleaned with sandpaper and then connected as shown below. The bulb lit up.
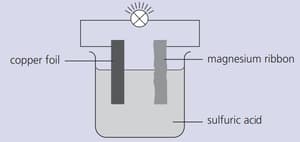
Name the electrolyte used.


Important Questions on Energy Changes, and Reversible Reactions
Strips of copper foil and magnesium ribbon were cleaned with sandpaper and then connected as shown below. The bulb lit up.
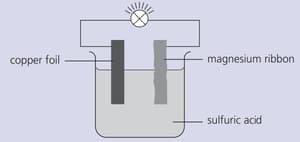
Explain why the bulb lit up.
Strips of copper foil and magnesium ribbon were cleaned with sandpaper and then connected as shown below. The bulb lit up.
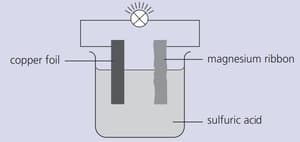
Which metal releases electrons into the circuit?
Strips of copper foil and magnesium ribbon were cleaned with sandpaper and then connected as shown below. The bulb lit up.
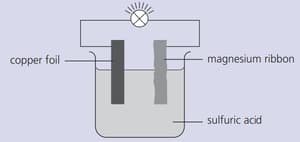
In this arrangement, energy is being changed from one form to another. Explain.
Strips of copper foil and magnesium ribbon were cleaned with sandpaper and then connected as shown below. The bulb lit up.
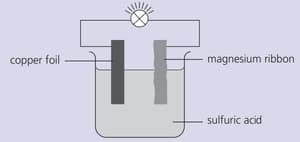
What is this type of arrangement called?
Strips of copper foil and magnesium ribbon were cleaned with sandpaper and then connected as shown below. The bulb lit up.
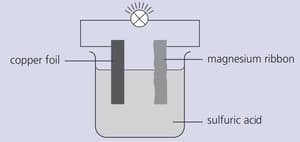
Give reasons why the set-up shown above would not be used as a torch battery.
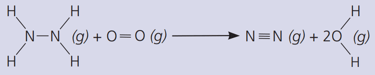
Count and list the bonds broken in this reaction.
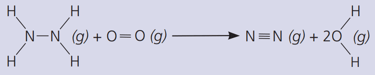
Count and list the new bonds formed.
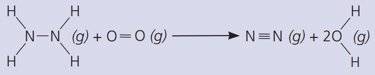
Calculate the total energy required to break the bonds.
The bond energies in are :
