HARD
JEE Main
IMPORTANT
Earn 100
Study the following figure and choose the correct options.
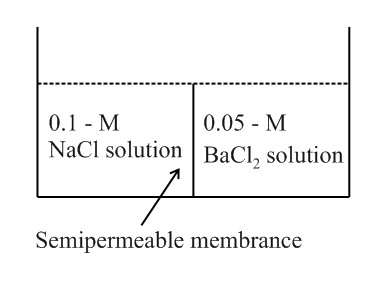

(a)There will be no movement of any solution across the membrane.
(b) will flow towards the solution.
(c)The osmotic pressure of is higher than the osmotic pressure of assuming the complete dissociation of the electrolyte.
(d) will flow towards the solution.
64.71% studentsanswered this correctly

Important Questions on Solutions
HARD
JEE Main
IMPORTANT
HARD
JEE Main
IMPORTANT
EASY
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
HARD
JEE Main
IMPORTANT
At , a solution containing of polyisobutylene in of benzene, developed a rise of at osmotic equilibrium. Calculate the molecular weight (in the multiple of ) of polyisobutylene if the density of the solution is . Give your answer up to one decimal place after rounding-off.
HARD
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
