Study the given graph carefully and answer the following question.
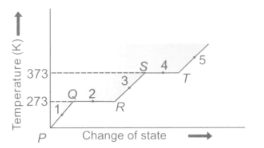
What is the physical state of matter at points and respectively?


Important Questions on Matter in Our Surroundings
Study the given graph carefully and answer the following question.
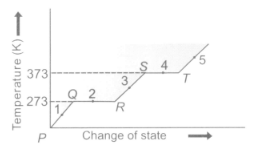
What is represented by straight lines and in the graph?
Sneha set up the experiment for melting of ice in a beaker and noted down the temperature throughout the process. What do you think she observed when the ice was melting?
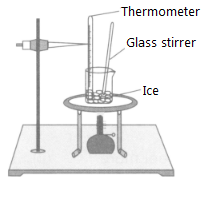
Which of the following statements are correct?
I. Temperature changes during the change of a state.
II. Dry ice gets converted directly into gaseous state under normal atmospheric conditions.
III. Higher boiling point of liquid indicates weaker intermolecular forces.
IV. Latent heat of vaporisation is generally higher than the latent heat of fusion for a substance.
Read the given statements and mark the correct option.
Statement 1: Solid changes its state when exposed to air.
Statement 2: undergoes sublimation.
Match column I with column II and choose the correct option from the given codes.
| Column I | Column I | ||
| P. | Hot tea poured in saucer gets cooled faster. | (i) | Evaporation increases with increase in temperature. |
| Q. | We feel more cold after a hot water bath than a cold water bath. | (ii) | Evaporation increases with decrease in humidity. |
| R. | Water is cool in earthen pitcher during dry hot day. | (iii) | Evaporation increases with increase in wind speed. |
| S. | We feel comfortable under a moving fan in summer. | (iv) | Evaporation increases with increase in surface area. |
Which of the following curves would be obtained on heating solid naphthalene to a temperature which is above its melting point?
