Suggest a reason the reaction between manganese carbonate and dilute hydrochloric acid speeds up when some concentrated hydrochloric acid is added.

Important Questions on The Speed of a Reaction
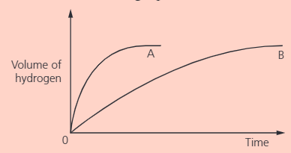
In two separate experiments, two metals and were reacted with an excess of dilute hydrochloric acid. The volume of hydrogen was measured every seconds. These graphs show the results:
Which piece of apparatus can be used to measure the volume of hydrogen produced?
In two separate experiments, two metals and were reacted with an excess of dilute hydrochloric acid. The volume of hydrogen was measured every seconds. These graphs show the results:
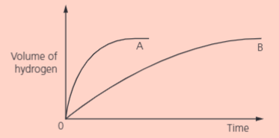
What measuring equipment is needed other than gas syringe?
In two separate experiments, two metals and were reacted with an excess of dilute hydrochloric acid. The volume of hydrogen was measured every seconds. These graphs show the results:
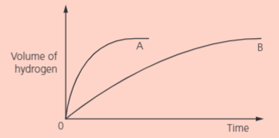
Which metal, and , reacts faster with hydrochloric acid? Give your evidence.
IN two separate experiments, two metals and were reacted with an excess of dilute hydrochloric acid. The volume of hydrogen was measured every seconds. These graphs shows the results:
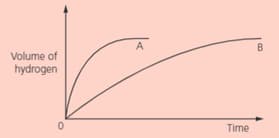
Sketch and label the curves that will be obtained for metal if more concentrated acid is used (curve ).
IN two separate experiments, two metals and were reacted with an excess of dilute hydrochloric acid. The volume of hydrogen was measured every seconds. These graphs shows the results:
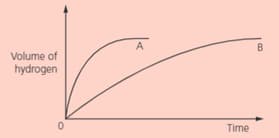
Sketch and label the curves that will be obtained for metal if the reaction is carried out at a lower temperature (curve ).
Copper(II) oxide catalyses the decomposition of hydrogen peroxide. of the oxide was added to a flask containing of hydrogen peroxide solution. A gas was releases. It was collected, and its volume noted every seconds. This table shows the results:
| Time / | ||||||||||
| Volume / |
What is a catalyst?
Copper(II) oxide catalyses the decomposition of hydrogen peroxide. of the oxide was added to a flask containing of hydrogen peroxide solution. A gas was releases. It was collected, and its volume noted every seconds. This table shows the results:
| Time / | ||||||||||
| Volume / |
Draw a diagram of suitable apparatus for this experiment.