Suggest how the experiment for the reaction between methanol and hydrochloric acid might be re-designed to obtain evidence for the effect of changing the concentration whilst controlling the concentration.

Important Questions on Reaction Kinetics
Nitrogen(V) oxide, , decomposes to nitrogen(IV) oxide and oxygen. . The table given below shows how the initial rate of reaction varies with the initial concentration of .
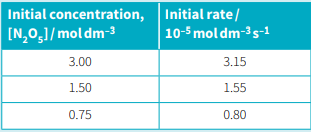
State the order of reaction for the decomposition of nitrogen(V) oxide.
Nitrogen(V) oxide, , decomposes to nitrogen(IV) oxide and oxygen. . The table given below shows how the initial rate of reaction varies with the initial concentration of .
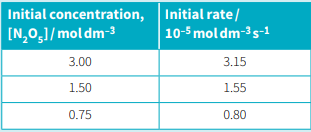
Use the data for in table to calculate a value for the rate constant for this decomposition.
Write the rate equation for the acid-catalysed reaction of iodine with propanone.
The equation below describes the reaction of propanone with iodine. Hydrogen ions catalyse this reaction. . The progress of the reaction can be followed by using a calorimeter. The brown colour of the iodine fades as the reaction proceeds. The experimental results are shown in Table below.
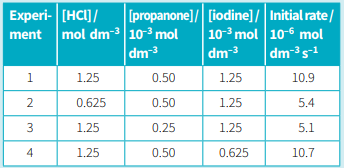
Use your rate equation and the information in Table (experiment 1) to calculate a value for the rate constant for this reaction.
An acidified solution of hydrogen peroxide reacts with iodide ions.
The rate equation for this reaction is rate . The mechanism below has been proposed for this reaction.
Explain why this mechanism is consistent with the rate equation.
The reaction is carried out in a sealed tube. At the start of the experiment, the concentration of both and , was The reaction is second order with respect to and first order with respect to . Which one of these represents the rate of reaction when the concentration of falls to ?
State whether the below given pair of substances might catalyse the reaction:
Explain your answer.
Given : of is and of is .
State whether the below given pair of substances might catalyse the reaction:
Explain your answer.
Given : of is and of is .
