MEDIUM
JEE Main
IMPORTANT
Earn 100
Sulphide ion in an alkaline solution reacts with solid sulphur to form polysulphide ions having formulacalculate for .

Important Questions on Chemical Equilibrium
EASY
JEE Main
IMPORTANT
Predict which of the following reaction will have an appreciable concentration of reactants and products:
,
EASY
JEE Main
IMPORTANT
Predict which of the following reaction will have an appreciable concentration of reactants and products:
, .
EASY
JEE Main
IMPORTANT
Predict which of the following reaction will have an appreciable concentration of reactants and products:
,
MEDIUM
JEE Main
IMPORTANT
Determine for the reaction, , from the following data at .
MEDIUM
JEE Main
IMPORTANT
For the reaction, , the dissociation pressure is at and . What will be the dissociation pressure at ?
MEDIUM
JEE Main
IMPORTANT
The variation of with is shown in the following graph. for the reaction will be:
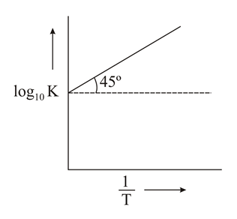
MEDIUM
JEE Main
IMPORTANT
For the following isomerisation reaction:
Cyclohexane Hexene .
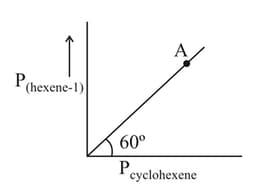
Which of the following statements holds good at point ?
HARD
JEE Main
IMPORTANT
For the chemical equilibrium, can be determined from which one of the following graphs?
