HARD
JEE Main
IMPORTANT
Earn 100
Sulphurous acid has and The of is____________ Round off to the Nearest Integer.
50% studentsanswered this correctly

Important Questions on Equilibrium
MEDIUM
JEE Main
IMPORTANT
Two salts and have the same value of solubility product of . The ratio of their molar solubilizes i.e. ________.
(Round off to the Nearest Integer).
MEDIUM
JEE Main
IMPORTANT
The solubility product of at is The concentration of hydroxide ions in a saturated solution of will be
MEDIUM
JEE Main
IMPORTANT
In the figure shown below reactant (represented by square) is in equilibrium with product (represented by circle). The equilibrium constant is (approx):
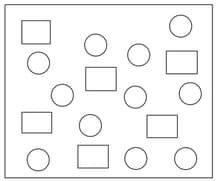
MEDIUM
JEE Main
IMPORTANT
The for the following dissociation is
Which of the following choices is correct for a mixture of and
EASY
JEE Main
IMPORTANT
Consider the following reversible chemical reactions:
.....(1)
.....(2)
The relation between and is:
MEDIUM
JEE Main
IMPORTANT
of solution is added to of solution. The of the resultant mixture is:
HARD
JEE Main
IMPORTANT
In an acid-base titration, solution was added to the solution of unknown strength. Which of the following correctly shown the change of of the titration mixture in this experiment?
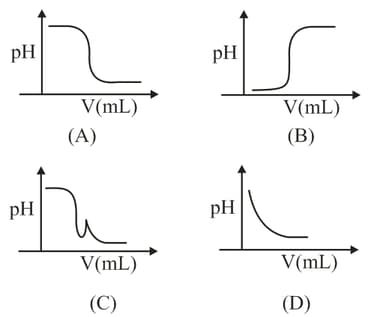
MEDIUM
JEE Main
IMPORTANT
of ammonia solution is treated with . If of ammonia solution is , the of the mixture will be:
