MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
Suppose the potential energy between electron and proton at a distance is given by Application of Bohr's theory to hydrogen atom in this case shows that
(a)energy in the orbit is proportional to
(b)energy is proportional to
(c)energy in the nth orbit is proportional to
(d)energy is proportional to
50% studentsanswered this correctly

Important Questions on Atomic Physics
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
The energy levels of a hypothetical one electron atom are shown in the figure
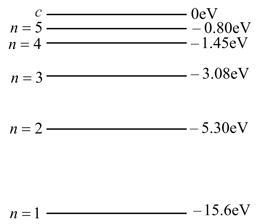
EASY
JEE Main/Advance
IMPORTANT
