Table gives the masses (in u) of several particles. . Use the table to determine to three significant figures:
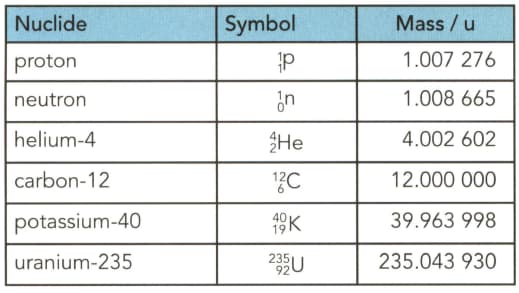
(a) the mass in $\mathrm{kg}$ of a helium- 4 nucleus.

Important Questions on Nuclear Physics
Table gives the masses (in u) of several particles. . Use the table to determine to three significant figures:
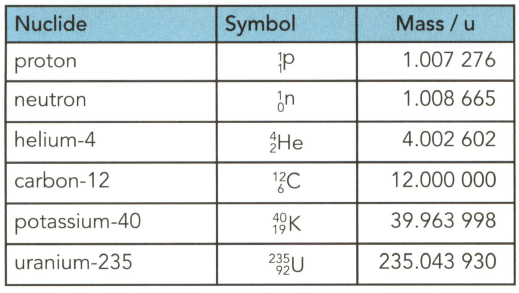
(b) the mass in gram (g) of $1.0$ mole of uranium-235 nuclei.
A nucleus of beryllium- decays into an isotope of boron by $\beta^{-}$ emission. The chemical symbol for boron is $\mathrm{B}$.
(a) Write a nuclear decay equation for the nucleus of beryllium-10.
A nucleus of beryllium- decays into an isotope of boron by $\beta^{-}$ emission. The chemical symbol for boron is $\mathrm{B}$.
(b) Calculate the energy released in this decay and state its form.
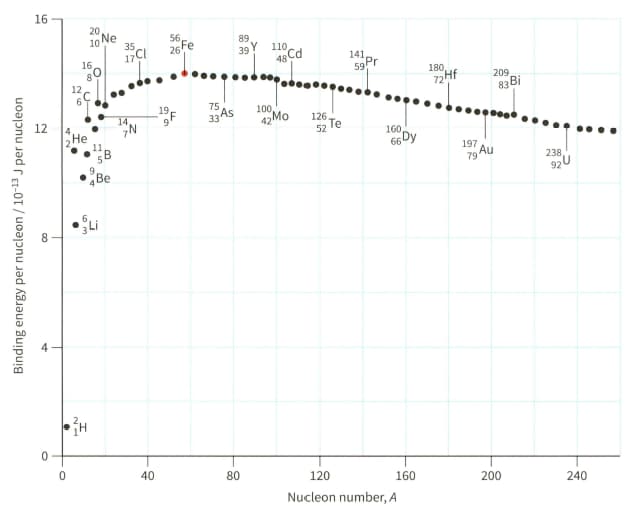
(a) Explain why hydrogen (proton) cannot appear on the graph shown in Figure.
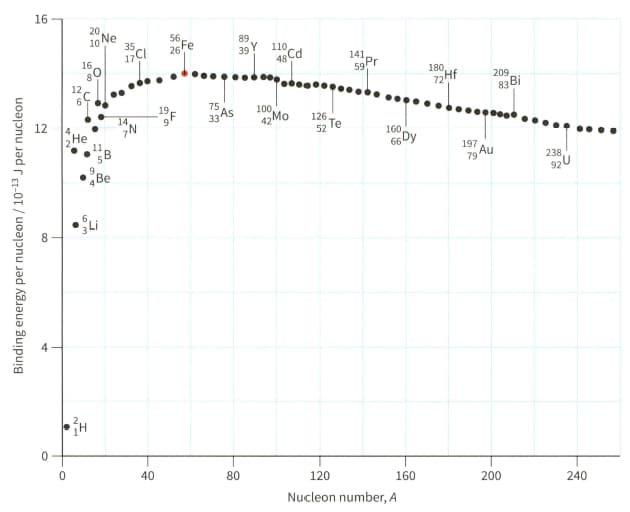
(b) Use Figure to estimate the binding energy of the nuclide .
The mass of Be nucleus is . For the nucleus of , determine:
(a) the mass defect in $\mathrm{kg}$
The mass of Be nucleus is . For the nucleus of , determine:
(b) the binding energy of the nucleus in $\mathrm{MeV}$
The mass of Be nucleus is . For the nucleus of , determine:
(c) the binding energy (in $\mathrm{MeV}$ ) per nucleon for the nucleus.
