EASY
MHT-CET
IMPORTANT
Earn 100
Temperature is a measurement of coldness or hotness of an object. This definition is based on
(a)Zeroth law of thermodynamics.
(b)First law of thermodynamics.
(c)Second law of thermodynamics.
(d)Newton's law of cooling.
44.44% studentsanswered this correctly

Important Questions on Kinetic Theory of Gases and Radiation
EASY
MHT-CET
IMPORTANT
A system is neither in thermal equilibrium with nor with . The systems and :
MEDIUM
MHT-CET
IMPORTANT
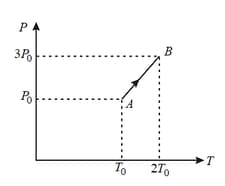
Pressure versus temperature graph of an ideal gas is as shown in figure. Density of the gas at point is . Density at point will be:
MEDIUM
MHT-CET
IMPORTANT
EASY
MHT-CET
IMPORTANT
If denote respectively the heat added, change in internal energy and the work done in a closed cycle process, then,
MEDIUM
MHT-CET
IMPORTANT
MEDIUM
MHT-CET
IMPORTANT
MEDIUM
MHT-CET
IMPORTANT
Assertion: Thermodynamic processes in nature are irreversible.
Reason: Dissipative effects cannot be eliminated.
EASY
MHT-CET
IMPORTANT
Assertion: In an isolated system, the entropy increases.
Reason: The processes in an isolated system are adiabatic.
