The 4f level is successively filled up in
Important Questions on Classification of Elements and Periodicity in Properties
Assertion: For hydrogenation reactions, the catalytic activity increases from Group to Group metals with maximum activity shown by Group elements.
Reason: The reactants are most strongly adsorbed on group elements.
Match the element in column I with that in column II.
| Column-I | Column-II | ||
| (a) | Copper | (p) | Non-metal |
| (b) | Fluorine | (q) | Transition metal |
| (c) | Silicon | (r) | Lanthanoid |
| (d) | Cerium | (s) | Metalloid |
Identify the correct match :
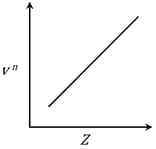
It is observed that characteristic X-ray spectra of elements show regularity. When frequency to the power 'n' i.e. of X-rays emitted is plotted against atomic number , the following graph is obtained.
The value of 'n' is
Match List - I with List - II
| LIST-I (Atomic number) |
LIST-II (Block of periodic table) |
||
| (A) | I. | -block | |
| (B) | II. | -block | |
| (C) | III. | -block | |
| (D) | IV. | -block | |
Choose the correct answer from the options given below:
Consider the following radioactive decays
I. and
II.
In which case group of parent and daughter elements remains unchanged.
(atomic number: , ,
The correct match among the following is
(a) Lithium, Sodium, Potassium (i) Alkaline earth metals
(b) Beryllium, Magnesium, Calcium (ii) Semi-metals
(c) Oxygen, Sulphur, Selenium (iii) Alkali metaIs
(d) Silicon, Germanium, Arsenic (iv) Chalcogens
Given Frequency of X-ray emitted
Atomic number