EASY
Earn 100
The CFSE (in Dq) and the spin only magnetic moment of metal ions in an octahedral environment is plotted below as a function of number of electrons.
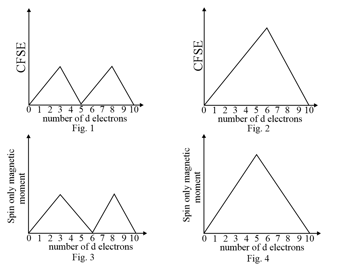

(a)2 and 4 represent strong field complexes
(b)1 and 4 represent weak field complexes
(c)2 and 3 represent strong field complexes
(d)1 and 3 represent weak field complexes
50% studentsanswered this correctly
Important Questions on Coordination Compounds
HARD
MEDIUM
MEDIUM
HARD
MEDIUM
HARD
MEDIUM
MEDIUM
MEDIUM
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
(Atomic No. = Ti : 22, Cr : 24 and Mo : 42)
MEDIUM
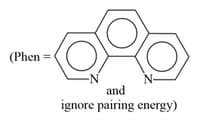
MEDIUM
and
absorb light in the visible region. The correct order of the wavelength of light absorbed by them is:
MEDIUM
EASY
MEDIUM
EASY

