The of a reaction in presence of a catalyst is and in absence of a catalyst is . What is slope of the plot of in the absence of catalyst

Important Questions on Chemical Kinetics
The reaction of hydrogen and iodine monochloride is represented by the equation:
This reaction is first-order in and also first-order in . Which of these proposed mechanisms
can be consistent with the given information about this reaction?
Mechanism :
Mechanism :
Following mechanism has been proposed for a reaction.
Step (slow)
Step (Fast)
The rate law expression for the reaction is:
The following mechanism has been proposed for a reaction:
The rate law expression for the reaction is:
(slow)
(fast)
The order of the overall reaction is
The reaction of and is first order in and
The reaction can take place by mechanism:
Select correct mechanism.
A substance undergoes first order decomposition. The decomposition follows two parallel first order reactions as :
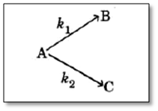
The percentage distribution of and are
