The Formation of precipitate in a reaction is represented in the chemical equation by a/an
Important Questions on Chemical Reactions and Equations
The values of stoichiometric coefficients and in the following reaction after balancing are, respectively:
In balanced chemical equation
A student was performing an experiment to understand the enzyme-substrate reaction. The student measured the formation of coloured product using a colorimeter. The student plotted the graph below which shows the reaction rate versus the substrate concentration.
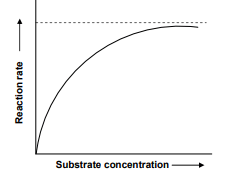
Following interpretations were drawn by the student:
A. The higher concentration of substrate acts as an enzyme inhibitor.
B. It is sigmoidal curve with sharp transition from low to high reactions rates over the increasing substrate concentration.
C. The curve reaches a plateau and does not further increase with increasing substrate concentrations due to saturation of enzyme with the substrate.
Choose which of the interpretations of the graph are correct.
In a balanced chemical equation
Which the set of coefficients that balance the equation.
Which of the following alternative is correct?
If moles of is mixed with moles of the maximum number of moles of formed will be
Write values of a, b, and c so that following chemical equation is balanced

