MEDIUM
MYP:4-5
IMPORTANT
Earn 100
The is a constant for the extent that pure water molecules dissociates into its component ions, protons and hydroxide ions . The value is the product of the hydrogen ion and hydroxide ion concentrations (or alternatively the square of the hydrogen ion concentration).
Table compares the extent that pure water molecules dissociates at different temperatures, and very precise measurements of pH values.
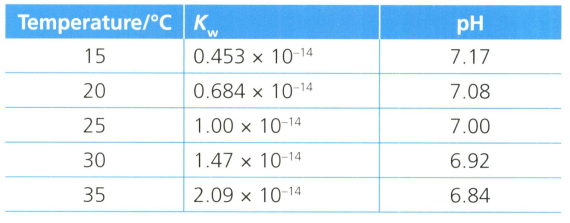
Explain why the pH changes in response to the dissociation of water molecules. What does pH measure?


Important Questions on What are the Impacts of Chemical Industry?
MEDIUM
MYP:4-5
IMPORTANT
Use the Bronsted-Lowry acid-base theory to write an equation showing how to water molecules react together and produce ions.
MEDIUM
MYP:4-5
IMPORTANT
As the number of ions present in the pure water changes with temperature, can we water still be considered neutral? Explain your reasoning.
