The Sun releases vast amounts of energy. Its power output is . Estimate how much its mass decreases each second because of this energy loss.

Important Questions on Nuclear Physics
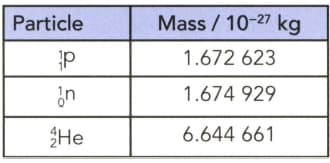
(a) Calculate the energy released if a nucleus is formed from separate stationary protons and neutrons. The masses of the particles are given in Table.
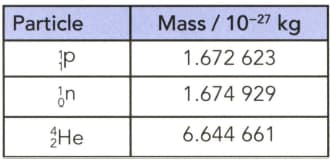
(b) Calculate also the energy released per nucleon.
The rest mass of a golf ball is .
Calculate its increase in mass when it is travelling at . What is this as a percentage of its rest mass?
Table gives the masses (in u) of several particles. . Use the table to determine to three significant figures:
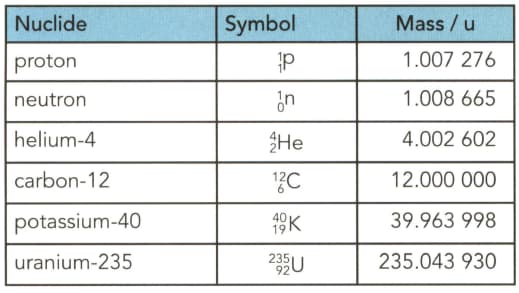
(a) the mass in $\mathrm{kg}$ of a helium- 4 nucleus.
Table gives the masses (in u) of several particles. . Use the table to determine to three significant figures:
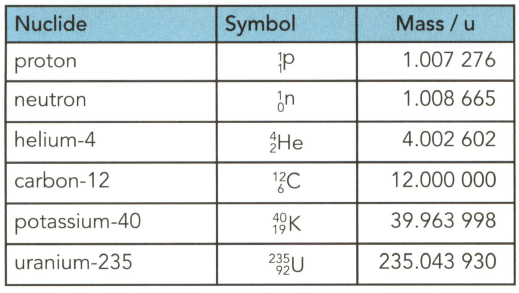
(b) the mass in gram (g) of $1.0$ mole of uranium-235 nuclei.
A nucleus of beryllium- decays into an isotope of boron by $\beta^{-}$ emission. The chemical symbol for boron is $\mathrm{B}$.
(a) Write a nuclear decay equation for the nucleus of beryllium-10.
