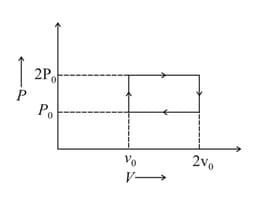
The above p-v diagram represents the thermodynamic cycle of an engine, operating with an ideal mono atomic gas. The amount of heat, extracted from the source in a single cycle is

Important Questions on Thermodynamics
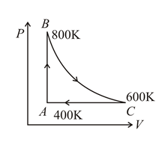
One mole of diatomic ideal gas undergoes a cyclic process as shown in figure. The process is adiabatic. The temperatures at and are and , respectively. Choose the correct statement.
A solid of constant heat capacity is being heated by keeping it in contact with reservoirs in two ways :
(i) Sequentially keeping in contact with 2 reservoirs such that each reservoir supplies same amount of heat.
(ii) Sequentially keeping in contact with 8 reservoirs such that each reservoir supplies the same amount of heat. In both the cases body is brought from initial temperature to final . Entropy change of the body in the two cases respectively is :
A thermally insulated, the closed copper vessel contains water at . When the vessel is shaken vigorously for , the temperature rises to . The mass of the vessel is and that of the water is . The specific heat capacities of copper and water are and , respectively. Neglect any thermal expansion.
How much heat is transferred to the liquid-vessel system?
How much work has been done on this system?
How much is the increase in internal energy of the system?
One gram of water becomes of steam when boiled at a pressure of one atmosphere. Latent heat of vaporization at this pressure is . Compute the work done.
Two moles of an ideal monoatomic gas are contained in a vertical cylinder of cross-sectional area as shown in the figure. The piston is frictionless and has a mass . At a certain instant a heater starts supplying heat to the gas at a constant rate . Find the steady velocity of the piston under isobaric condition. All the boundaries are thermally insulated.