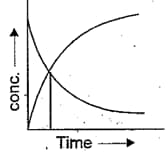
The accompanying figure depicts the change in concentration of species and for the reaction as a function of time, the point of intersection of the two curves represents


Important Questions on Chemical Kinetics
The rate law for the dimerisation of into is
Which of the following changes will change the value of the specific rate constant
The order of a reaction product in which half the reagent is reacted is half an hour, three quarters in one hour and seven - eighth in one and half hours is
The frequency factor has the same unit as the rate constant, .
A plot rate vs for the order reaction gives a straight line with stope intercept in.
The order of a reaction product in which half the reagent is reacted in half an hour, three quarters in one hour and seven - eighth in one and half hours must be (unity).
The unit of rate constant for a second order reaction will be is representing the molarity of solution.)
For the reaction
the following mechanism has been given
Hence, rate law is:
