HARD
JEE Main
IMPORTANT
Earn 100
The activation energy of one of the reactions in a biochemical process is . When the temperature falls from to , the change in rate constant observed is . The value of is
[Given: ]
25% studentsanswered this correctly

Important Questions on Chemical Kinetics
MEDIUM
JEE Main
IMPORTANT
The equation is followed for the decomposition of compound . The activation energy for the reaction is ____ . [nearest integer]
(Given: )
MEDIUM
JEE Main
IMPORTANT
The half life for the decomposition of gaseous compound is when the gaseous pressure was Torr initially. When the pressure was Torr, the half life was found to be . The order of the reaction is _____ (Nearest integer)
MEDIUM
JEE Main
IMPORTANT
For the decomposition of azomethane. a first order reaction, the variation in partial pressure with time at is given as
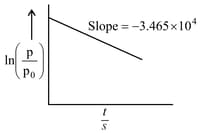
The half life of the reaction is____
MEDIUM
JEE Main
IMPORTANT
For a reaction the half lives are and when the concentration of reactant is and respectively. The order of the reaction is
HARD
JEE Main
IMPORTANT
At , the half life for the decomposition of is and is independent of the initial concentration of . The time required for of the to decompose is (Given: ; )
MEDIUM
JEE Main
IMPORTANT
The above reaction has been studied at . The related data are given in the table below
| Reaction serial number | Initial pressure of | Initial Pressure of |
Initial rate |
The order of the reaction with respect to NO is____
HARD
JEE Main
IMPORTANT
If formation of compound follows the first order of kinetics and after minutes the concentration of was found to be half of its initial concentration. Then the rate constant of the reaction is . The value of is____
HARD
JEE Main
IMPORTANT
For the given first order reaction the half life of the reaction is . The ratio of the initial concentration of reactant to the concentration of reactant at time will be equal to _____. (Nearest integer)
