MEDIUM
Earn 100
The additional voltage needed to carry out an electrochemical reaction at a certain rate is called overvoltage.
(a)True
(b)False
50% studentsanswered this correctly
Important Questions on Electrochemistry
HARD
MEDIUM
MEDIUM
A current of flows for through an electrolytic cell containing a molten salt of metal . This results in the decomposition of of metal at the cathode. The oxidation state of in the molten salt is:
EASY
MEDIUM
Some materials are given below:
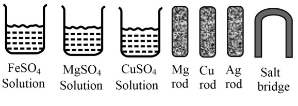
Write the chemical equation fo the reaction taking place at the cathode.
HARD
MEDIUM
MEDIUM
EASY
EASY
MEDIUM
EASY
MEDIUM
MEDIUM
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
Some materials are given below:
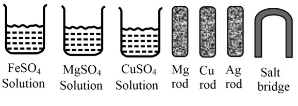
Which is the anode of this cell?
HARD

