EASY
JEE Main/Advance
IMPORTANT
Earn 100
The adiabatic exponent of a gas is equal to :
(a).
(b).
(c)
(d)
100% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
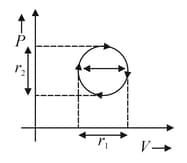
A sample of gas is going through cyclic process, work done by gas is
EASY
JEE Main/Advance
IMPORTANT
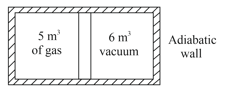
A movable piston separates a sample of gas from vacuum. In this state, pressure of gas is , then find pressure after piston is released.
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
