MEDIUM
Earn 100
The alkali metal that reacts with nitrogen directly to form nitride is
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on The s-Block Elements
EASY
MEDIUM
Reaction of with gives :
(A)
(B)
(C)
(D)
(E)
Choose the correct answer from options given below
EASY
EASY
Match list-I with list-II :
| (a) | (i) | Treatment of cancer | |
| (b) | (ii) | Extraction of metals | |
| (c) | (iii) | Incendiary bombs and signals | |
| (d) | (iv) | Windows of X-ray tubes | |
| (v) | Bearings for motor engines. |
Choose the most appropriate answer, the option given below :
EASY
EASY
MEDIUM
HARD
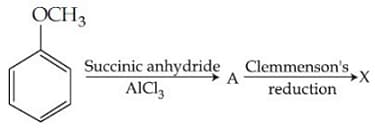
X is :
MEDIUM
HARD
EASY
Match List with List
| List | List | ||
| (a) | (i) | Antacid | |
| (b) | (ii) | Cement | |
| (c) | (iii) | Bleach | |
| (d) | (iv) | Plaster of paris |
Choose the most appropriate answer from the
MEDIUM
EASY
EASY
EASY
EASY
EASY
MEDIUM
EASY
MEDIUM

