MEDIUM
8th ICSE
IMPORTANT
Earn 100
The atom of an element is made up of 4 protons, 5 neutrons and 4 electrons. Write down its atomic number and its mass number.

Important Questions on Atomic Structure
HARD
8th ICSE
IMPORTANT
Sodium atom (Atomic number 11; Mass No. 23)
MEDIUM
8th ICSE
IMPORTANT
Draw the diagrams representing the atomic structure of the following:
Chlorine atom (Atomic number 17; Mass No. 35).
MEDIUM
8th ICSE
IMPORTANT
Draw the diagram representing the atomic structure of the following:
Carbon atom (Atomic number 6; Mass No. 12).
MEDIUM
8th ICSE
IMPORTANT
MEDIUM
8th ICSE
IMPORTANT
HARD
8th ICSE
IMPORTANT
HARD
8th ICSE
IMPORTANT
HARD
8th ICSE
IMPORTANT
The electronic configuration of two elements X and Y are given below
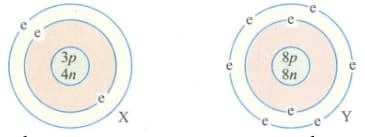
where p = proton, n = neutron, e = electron. When these two elements combine together to form a compound, give the mass (in grams) of one mole of this compound.
