The atomic radii of the elements of the second period are given below:
Second period elements
B
Be
O
N
LI
F
C
Atomic radii (pm)
88
111
66
74
152
64
77
Arrange these elements in decreasing order of their atomic radii.

Important Questions on Periodic Classification of Elements
The atomic radii of the elements of the second period are given below:
| Second period elements | B | Be | O | N | Li | F | C |
| Atomic radii (pm) | 88 | 111 | 66 | 74 | 152 | 64 | 77 |
Are the elements now arranged in the pattern of a period in the periodic table?
The atomic radii of the elements of the second period are given below:
| Second period elements | B | Be | O | N | Li | F | C |
| Atomic radii (pm) | 88 | 111 | 66 | 74 | 152 | 64 | 77 |
Name the element which has the (a) largest and (b) the smallest atomic number.
The atomic radii of the elements of the second period are given below:
| Second period elements | B | Be | O | N | Li | F | C |
| Atomic radii (pm) | 88 | 111 | 66 | 74 | 152 | 64 | 77 |
From the above data, infer how the atomic size or atomic radius of the elements change as we go from left to right in a period.
The atomic radii of the elements of the second period are given below:
| Second period elements | B | Be | O | N | Li | F | C |
| Atomic radii (pm) | 88 | 111 | 66 | 74 | 152 | 64 | 77 |
Name one metal, one nonmetal, and a metalloid out of these elements.
The atomic radii of the elements of the second period are given below:
| Second period elements | B | Be | O | N | Li | F | C |
| Atomic radii (pm) | 88 | 111 | 66 | 74 | 152 | 64 | 77 |
Why does atomic radius decrease as we move from left to right in a period?
Observe the following part of the Periodic Table and answer the question.
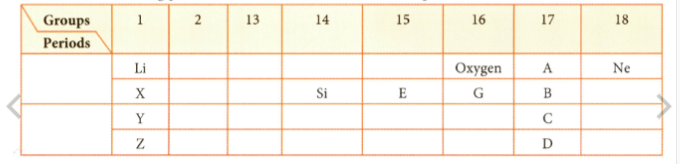
Which is the most reactive metal?
Observe the following part of the Periodic Table and answer the question.
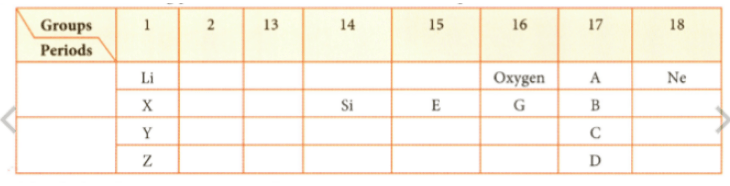
Which is the most reactive nonmetal?
Observe the following part of the Periodic Table and answer the question.
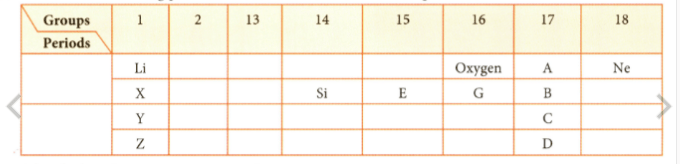
Name the family of A, B, C, and D.
