HARD
Chemistry
IMPORTANT
Earn 100
The average bond energy is , first I.E. of is , electron affinity of is and bond dissociation energy of is . Then, the enthalpy change for the reaction
will be
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Thermodynamics
HARD
Chemistry
IMPORTANT
EASY
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
EASY
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
HARD
Chemistry
IMPORTANT
Select the correct statement for the equilibrium under standard conditions:
HARD
Chemistry
IMPORTANT
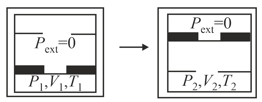
For an ideal gas, consider only work in going from an initial state to the final state . The final state can be reached by either of the two paths shown in the figure. Which of the following choice(s) is(are) correct? [Take as change in entropy and as work done.]