The average atomic mass of magnesium is This magnesium is composed of of and remaining of and Calculate of

Important Questions on Some Basic Concepts of Chemistry
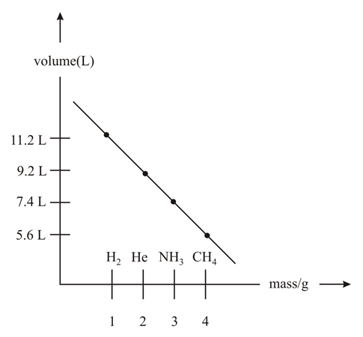
Which of the following atoms/molecules are placed at the correct position?
of dry green algae absorbs moles of per hour by photosynthesis. If the fixed carbon atoms were all stored after photosynthesis as starch, , how long (in hours) would it take for the algae to double their own weight assuming photosynthesis takes place at a constant rate?
Round-off your answer to two places of decimal.
A plant virus is found to consist of uniform cylindrical particles of in diameter and long. The specific volume of the virus is . If the virus is considered to be a single particle, find its molecular weight.
If the answer is of type , report the value of correct up to two places of decimals.
When a mixture of and is repeatedly digested with sulphuric acid, all the halogens are expelled and is formed quantitatively. With a particular mixture, it was found that the weight of obtained was precisely the same as the weight of mixture taken. Calculate the ratio of the weights of and in the mixture.
Give the final answer up to two places of decimal.
