MEDIUM
NEET
IMPORTANT
Earn 100
The average kinetic energy of a gas molecule at is Its average kinetic energy at will be
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Kinetic Theory
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
HARD
NEET
IMPORTANT
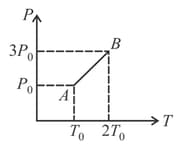
Pressure versus temperature graph of an ideal gas is as shown in figure. Density of the gas at point is Density at point will be