EASY
MHT-CET
IMPORTANT
Earn 100
The average kinetic energy per molecule of an ideal gas at is:
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on States of Matter
EASY
MHT-CET
IMPORTANT
If the temperature of mole of a gas is increased by , then the change in kinetic energy of the system will be:
EASY
MHT-CET
IMPORTANT
If the RMS velocity of one mole of helium gas having molar mass is , what is the correct expression for ?
(Average kinetic energy is )
EASY
MHT-CET
IMPORTANT
If the of a gas at is , then the molar mass of the gas is:
MEDIUM
MHT-CET
IMPORTANT
On increasing the temperature from to , the average kinetic energy of argon gas increases by a factor of:
EASY
MHT-CET
IMPORTANT
The pressure of moles of gas in a flask at a certain temperature is . The temperature of the gas is:
(Given: )
EASY
MHT-CET
IMPORTANT
The compression factor (compressibility factor) for one mole of a Van der Waal's gas at and is found to be . Assuming that the volume of the gas molecule is negligible, what is the value of the Van der Waal's constant ?
MEDIUM
MHT-CET
IMPORTANT
A gas shows positive deviation and obeys the equation of state . The slope and intercept of the isobaric curve are:
MEDIUM
MHT-CET
IMPORTANT
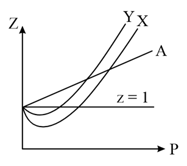
Which one of the following is most likely to be correct regarding the graph given above?
