The boiling point of water on the Fahrenheit scale is .
Important Questions on Thermal Properties of Matter
Convert into Celsius.
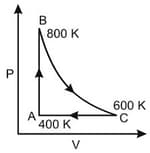
One mole of diatomic ideal gas undergoes a cyclic process ABC as shown in figure. The process BC is adiabatic. The temperatures at A, B and C are 400 K, 800 K and 600 K respectively. Choose the correct statement :
Consider a ball of mass attached to one end of a spring and immersed in water. Assume the complete system is in thermal equilibrium. The spring is now stretched to and the mass is released so that it vibrates up and down. Estimate the change in temperature of water before the vibrations stop.
(Specific heat of the material of the ball and Specific heat of water )
Two rods A and B of different materials are welded together as shown in figure. Their thermal conductivities are and . The thermal conductivity of the composite rod will be