EASY
9th CBSE
IMPORTANT
Earn 100
The boiling points of diethyl ether, acetone and -butyl alcohol are and respectively. Which one of the following correctly represents their boiling points in Kelvin scale?
(a)
(b)
(c)
(d)
66.67% studentsanswered this correctly

Important Questions on Matter in Our Surroundings
EASY
9th CBSE
IMPORTANT
Which condition out of the following will increase the evaporation of water?
MEDIUM
9th CBSE
IMPORTANT
In which of the following conditions, the distance between the molecules of hydrogen gas would increase?
(i) Increasing pressure on hydrogen contained in a closed container.
(ii) Some hydrogen gas leaking out of the container.
(iii) Increasing the volume of the container of hydrogen gas.
(iv) Adding more hydrogen gas to the container without increasing the volume of the container.
MEDIUM
9th CBSE
IMPORTANT
MEDIUM
9th CBSE
IMPORTANT
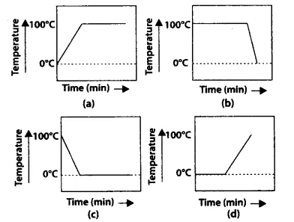
A student heats a beaker containing ice and water. He measures the temperature of the content of the beaker as a function of time. Which of the following figure would correctly represent the result? Justify your choice.
EASY
9th CBSE
IMPORTANT
EASY
9th CBSE
IMPORTANT
EASY
9th CBSE
IMPORTANT
MEDIUM
9th CBSE
IMPORTANT
Match the physical quantities given in column A to their Sl units given in column B.
| Column (A) | Column (B) | ||
| (a) | Pressure | (i) | Cubic metre |
| (b) | Temperature | (ii) | kilogram |
| (c) | Density | (iii) | Pascal |
| (d) | Mass | (iv) | Kelvin |
| (e) | Volume | (v) | Kilogram per cubic metre |