EASY
AS and A Level
IMPORTANT
Earn 100
The bond angles around a carbon atom in which its atomic orbitals are hybridised is likely to be:
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Benzene and its Compounds
EASY
AS and A Level
IMPORTANT
How many electrons are involved in a pi bond?
EASY
AS and A Level
IMPORTANT
Describe the reaction mechanism when is reacted with ethene, .
EASY
AS and A Level
IMPORTANT
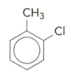
Discuss with a partner how you would name this compound:
EASY
AS and A Level
IMPORTANT
Discuss what happens in the substitution reaction that takes place between either chloroethane and or chloroethane and .
EASY
AS and A Level
IMPORTANT
Give the number of electrons involved in the bonding system in a benzene molecule.
EASY
AS and A Level
IMPORTANT
State the type of atomic orbital that the electrons involved in the bonding system in a benzene molecule.
EASY
AS and A Level
IMPORTANT
Explain what we mean by the term delocalised electrons in benzene.
EASY
AS and A Level
IMPORTANT
Compare the bonding in benzene with the bonding in hex--ene.