EASY
NEET
IMPORTANT
Earn 100
The bond energies for single covalent bonds of hydrogen atoms with elements and are and , respectively. The element which has the smallest atom is:
(a)
(b)
(c)
(d)
56.25% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
HARD
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
What is the correct order of the dipole moment for the compounds given below?
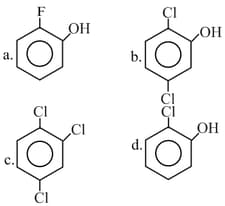
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
Which of the following is incorrect?
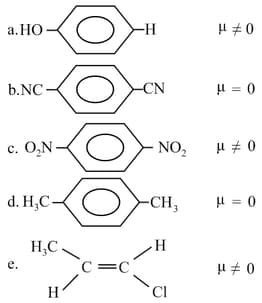
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
