EASY
Earn 100
The bond length decreases with bond multiplicity.
(a)True
(b)False
50% studentsanswered this correctly
Important Questions on Chemical Bonding and Molecular Structure
MEDIUM
MEDIUM
MEDIUM
MEDIUM
The bond lengths (in of and are:
MEDIUM
EASY
EASY
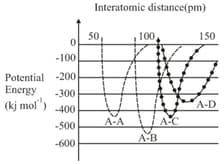
MEDIUM
MEDIUM
MEDIUM
MEDIUM
MEDIUM
Find out the correct order of ionic character in the following molecules
MEDIUM
EASY
How many ions of the following have a bond order of
EASY
EASY
EASY
EASY
EASY
EASY

