MEDIUM
JEE Main
IMPORTANT
Earn 100
The calculated spin-only magnetic moments of the anionic and cationic species out of and respectively, are:
(a) and
(b) and
(c) and
(d) and
9.09% studentsanswered this correctly

Important Questions on Coordination Compounds
MEDIUM
JEE Main
IMPORTANT
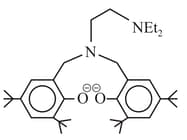
The following ligand is:
MEDIUM
JEE Main
IMPORTANT
The correct order of the spin –only magnetic moment of metal ions in the following low-spin complexes, and is:
HARD
JEE Main
IMPORTANT
Consider the following reaction and statements:
(i) Two isomers are produced if the reactant complex ion is a cis-isomer.
(ii) Two isomers are produced if the reactant complex ion is a trans-isomer.
(iii) Only one isomer is produced if the reactant complex ion is a trans-isomer.
(iv) Only one isomer is produced if the reactant complex ion is a cis-isomer.
The correct statements are
MEDIUM
JEE Main
IMPORTANT
The number of possible optical isomers for the complexes with and hybridized metal atom, respectively, is:
Note: and are unidentate neutral and unidentate monoanionic ligands, respectively.
EASY
JEE Main
IMPORTANT
The IUPAC name of the complex is
HARD
JEE Main
IMPORTANT
The theory that can completely/properly explain the nature of bonding in is:
MEDIUM
JEE Main
IMPORTANT
Which of the following complex species is not expected to exhibit optical isomerism?
MEDIUM
JEE Main
IMPORTANT
The octahedral complex of a metal ion with four monodentate ligands absorb wavelengths in the region of red, green, yellow and blue, respectively. The increasing order of ligand strength of the four ligands is
