EASY
Earn 100
The carbon atoms in are
(a) hybridised
(b) hybridised
(c) hybridised
(d) hybridised
50% studentsanswered this correctly
Important Questions on Some Basic Principles of Organic Chemistry
EASY
How many pi bonds and sigma bonds are present in following molecule?
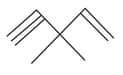
MEDIUM
Draw the structure of propanal.
MEDIUM
Draw the structural formula of butanone.
MEDIUM
The number of hybridised carbons in an acyclic neutral compound with molecular formula is
EASY
In molecule, the hybridization of carbon and respectively, are :
EASY
Give the structural formula of acetic acid.
EASY
Write the Fischer structure of Bromobutane.
EASY
The number of hybridized carbon atoms in
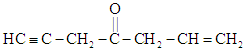
EASY
Which of the following is an incorrect statement?
MEDIUM
Give the structural formula of Methylpropanol.
MEDIUM
Which of the following structures contain -hybridised carbon atom(s)?
I.
II.
III. 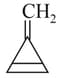
IV. 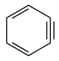
EASY
The number of C - C sigma bonds in the compound

EASY
In allene , the type(s) of hybridization of the carbon atoms is (are):
MEDIUM
Represent the union of two sets by Venn diagram for each of the following.
is a prime number between and
is an odd number between and
EASY
The hybridisation of carbon atoms in the C-C single bond of is:
EASY
The sum of the number of σ and π bonds in 5-oxohexanoic acid is:
MEDIUM
The hybridisation of carbon atoms in C-C single bond
HARD
Indicate the bonds in the following molecules:
(cyclic compound),
MEDIUM
What is the type of hybridisation of carbon atoms marked with star?
Select the correct statement regarding the same.
MEDIUM
In the dehydration reaction the hybridization state of carbon change from

