MEDIUM
Earn 100
The carbon-oxygen bond length in phenols is slightly less than in methanol. Give reasons.
Important Questions on Alcohols, Phenols and Ethers
HARD
Possible isomers of monohydric phenol having molecular formula are _____.
MEDIUM
Identify and respectively, in the following reaction sequence are
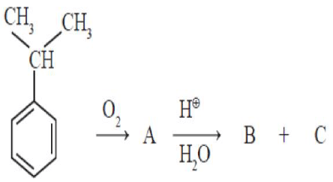
HARD
Draw the resonating structures of phenol and predict whether group is meta directing or ortho and para directing towards electrophilic ring substitution reactions.
MEDIUM
Write the structural formula and IUPAC name of three isomers of molecular formula .
EASY
Which of the following is/are aromatic alcohol?
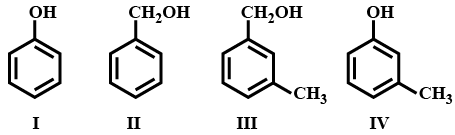
MEDIUM
A solution of bromine, in methanol or ethanol cannot be used for the detection of unsaturation in organic compounds. Why?
EASY
How many structural isomers of will be primary alcohols?
MEDIUM
The name of Diacetone alcohol is
EASY
The IUPAC name of dimethyl ether is
EASY
Which one of the following is optically active?
HARD
The correct combination of names for isomeric alcohols with molecular formula is/are
EASY
Which one of the following is a secondary alcohol?
MEDIUM
Which one of the following is a simple ether?
EASY
Which of the following does not contain carbon-oxygen double bonds?
EASY
The chemical name of anisole is
EASY
A Fischer projection of -butanediol is:
MEDIUM
How many metameric ethers are represented by the molecular formula
EASY
The IUPAC name of ethyl isobutyl ether is
EASY
General formula of primary alcohol is

