The chart shows the process of extraction of some metals.
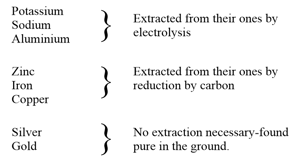 .
.
Which of these metals requires electricity for extraction from its ore?
 .
.
Important Questions on Metals and Non-metals
The table shows four different materials and their resistivity.
| Magnesium | Sulphur | |
| Number of electrons in the shells of the atom |
How many atoms of sulphur will react with one atom of magnesium to form a compound?
Listed here is the reactivity of certain metals:
| Metal | Reaction with air | Reaction with water | Reaction with dilute acids |
| Gold | Does not oxidise or burn | No reaction | No reaction |
| sodium | Burns vigorously to form an oxide | Reacts on heating | Reacts to produce hydrogen |
| Zinc | Burns to form an oxide | Reacts on heating | Reacts to produce hydrogen |
| platinum | No reaction | Does not dissolve or reacts | No reaction |
From the list above, identify the metal(s) that are likely to be found in a pure state in the Earth's Crust.
The blue-coloured solution of the sulphate salt of metal is taken in a beaker. Metal powders and are added one after the other to the beaker. The colour changes occurring in the solution are shown below:
State what colour change, if any, will occur if metal is again added to the green solution in the beaker. Explain Why.
A piece of iron rusts when it comes in contact with air and moisture. Prakash had two identical shiny iron pieces and . To prevent the pieces from rusting, he coated piece with oil paint and he galvanised piece with a coat of zinc metal. He noticed that the coatings were not complete and that a small part of the iron was exposed in both the pieces.
What is Prakash likely to observe about the exposed parts of the two iron pieces after some days? Explain why.
Stainless steel does not rust.
Iron, nickel and chromium form an alloy.
Does statement present a valid explanation for statement ? Justify your answer.
