EASY
8th Kerala Board
IMPORTANT
Earn 100
The chemical formula of sulphuric acid is . Altogether, how many atoms are present? What if it is ? Determine the total number of atoms present in the molecules given below:

Important Questions on Basic Constituents of Matter
EASY
8th Kerala Board
IMPORTANT
EASY
8th Kerala Board
IMPORTANT
EASY
8th Kerala Board
IMPORTANT
Zinc Hydrochloric acid Zinc chloride Hydrogen gas
Note down the reactants and the products in the above given reaction.EASY
8th Kerala Board
IMPORTANT
The reaction between zinc and hydrochloric acid can be represented as follows:
Now, see how the number of atoms on both sides of the arrow has been tabulated.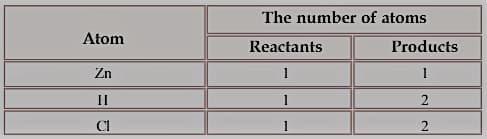
EASY
8th Kerala Board
IMPORTANT
Rewrite the equation as follows:
Examine the number of atoms in the equation.

EASY
8th Kerala Board
IMPORTANT
Now, look at the balanced chemical equations given below. Write down the reactants and products in the table:
(1)
(2)
(3)
| No. | Reactants | Products |
| 1. | _____ | _____ |
| 2. | _____ | _____ |
| 3. | _____ | _____ |
MEDIUM
8th Kerala Board
IMPORTANT
EASY
8th Kerala Board
IMPORTANT
Classify the following into elements and compounds.
Ammonia, sugar, nitrogen, mercury, sodium chloride, water, copper sulphate, sodium, carbon.
