MEDIUM
Earn 100
The compressor of a Carnot refrigerator consumes of energy per cycle. The hot reservoir is at temperature and the amount of energy released to it per cycle is . Then the lowest possible temperature attained by the cold reservoir will be close to
75% studentsanswered this correctly
Important Questions on Heat and Thermodynamics
EASY
MEDIUM
EASY
EASY
EASY
MEDIUM
EASY
HARD
The efficiency of the cycle shown below in the figure (consisting of one isobar, one adiabat and one isotherm) is The ratio, between the highest and lowest temperatures attained in this cycle obeys (the working substance is a ideal gas):-
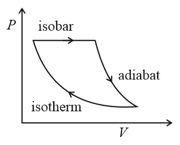
HARD
MEDIUM
MEDIUM
EASY
MEDIUM
EASY
EASY
EASY
For a Carnot engine which of the following is false?
EASY
EASY
MEDIUM
MEDIUM