The conductivity of 0.001 M Na2SO4 solution is 2.6 x 10-4 Scm-1 which increases to 7.0 x 10-4 Scm-1, when the solution is saturated with CaSO4. The molar conductivities of Na+ and Ca2+ are 50 and 120 Scm2mol-1, respectively. What is the solubility product of CaSO4 ? Neglect the conductivity of used water.
Important Questions on Electrochemistry
The variation of molar conductively with concentration of an electrolyte (X) in aqueous solution is shown in the given figure.
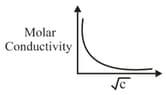
The electrolyte X is :
: Conductivity always increases with decreases in the concentration of electrolyte.
: Molar conductivity always increases with decreases in the concentration of electrolyte.
The correct option among the following
Given below are two statements:
Statement I : The limiting molar conductivity of (strong electrolyte) is higher compared to that of $\mathrm{CH}_{3} \mathrm{COOH}$ (weak electrolyte).
Statement II : Molar conductivity decreases with decrease in concentration of electrolyte.
In the light of the above statements, choose the most appropriate answer from the options given below:
Match List - I with List - II :
| List - I (Parameter) |
List - II (Unit) | ||
| (a) | Cell constant | (i) | |
| (b) | Molar conductivity | (ii) | Dimensionless |
| (c) | Conductivity | (iii) | |
| (d) | Degree of dissociation of electrolyte | (iv) |

