EASY
JEE Main
IMPORTANT
Earn 100
The correct decreasing order of energy, for the orbitals having, following set of quantum numbers:
(A)
(B)
(C)
(D)
(a)
(b)
(c)
(d)
83.33% studentsanswered this correctly

Important Questions on Structure of Atom
MEDIUM
JEE Main
IMPORTANT
Identify the incorrect statement from the following.
EASY
JEE Main
IMPORTANT
Which of the following pair is not isoelectronic species?
(Atomic numbers )
HARD
JEE Main
IMPORTANT
If the wavelength of an electron emitted from atom is , then energy absorbed by the electron in its ground state compared to the minimum energy required for its escape from the atom, is____times.
[Given : , Mass of electron ], Give an answer to the nearest integer value.
HARD
JEE Main
IMPORTANT
The minimum uncertainty in the speed of an electron in one dimensional region of length
(Where Bohr radius ) is____ (Nearest integer) (Given : Mass of electron , Planck's constant )
EASY
JEE Main
IMPORTANT
Given below are the quantum numbers for electrons.
A.
B.
C.
D.
The correct order of increasing energy is
MEDIUM
JEE Main
IMPORTANT
If wavelength of the first line of the Paschen series of hydrogen atom is , then the wavelength of the second line of this series is _______ . (Nearest integer)
MEDIUM
JEE Main
IMPORTANT
The number of s-electrons present in an ion with protons in its unipositive state is
EASY
JEE Main
IMPORTANT
Following figure shows spectrum of an ideal black body at four different temperatures. The number of correct statement/s from the following is _______.
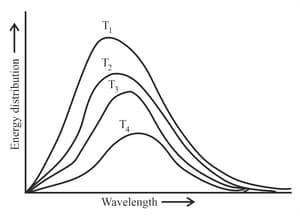
A. T4 > T3 > T2 > T1
B. The black body consists of particles performing simple harmonic motion.
C. The peak of the spectrum shifts to shorter wavelength as temperature increases.
D.
E. The given spectrum could be explained using quantisation of energy
