EASY
Earn 100
The correct order of boiling points of isomeric amines is
(a)Tertiary amines Secondary amines Primary amines
(b)Secondary amines Tertiary amines Primary amines
(c)Primary amines Tertiary amines
Secondary amines
Secondary amines
(d)Primary amines Secondary amines
Tertiary amines
Tertiary amines
50% studentsanswered this correctly
Important Questions on Amines
EASY
EASY
MEDIUM
MEDIUM
MEDIUM
Arrange the following in increasing order of boiling points :
EASY
MEDIUM
Account for the following :
Ethylamine is soluble in water whereas aniline is not.
EASY
Given below are two statements :
Statement I : Pure Aniline and other arylamines are usually colourless.
Statement II : Arylamines get coloured on storage due to atmospheric reduction.
In the light of the above statements, choose the most appropriate answer from the options given below :
MEDIUM
Give reasons : is more basic than in an aqueous solution.
MEDIUM
Arrange the following compounds in decreasing order of their boiling points:
Butanol, Butanamine, Butane
EASY
MEDIUM
MEDIUM
Explain the following :
Amines are less acidic than alcohols of comparable molecular masses.
EASY
EASY
MEDIUM
MEDIUM
MEDIUM
HARD
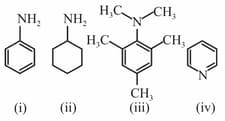
EASY

