HARD
JEE Advanced
IMPORTANT
Earn 100
The correct order of bond length between is
(a)
(b)
(c)
(d)
92.31% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
MEDIUM
JEE Advanced
IMPORTANT
The percentage of character in the orbitals forming bonds in is
MEDIUM
JEE Advanced
IMPORTANT
The correct order of increasing bond length of and is
MEDIUM
JEE Advanced
IMPORTANT
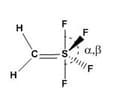
axial equatorial
Which of the following option is correct regarding and
MEDIUM
JEE Advanced
IMPORTANT
What is the correct order of bond angle of the following molecule?
HARD
JEE Advanced
IMPORTANT
Select the correct statement(s).
HARD
JEE Advanced
IMPORTANT
The incorrect order of the bond length in and is
HARD
JEE Advanced
IMPORTANT
The correct order of bond length follows the sequence:
HARD
JEE Advanced
IMPORTANT
Consider the following statements:
() Percentage of -bonding in bond follows the sequence .
() Relative strength of-bonding in , follows the sequence : .
() The correct order of bond length ( ) follows the sequence :
-bond order follows the sequence : .
Using '' for 'True' and '' for 'False' statement in the given sequence, pick the correct set of codes.
