MEDIUM
JEE Main
IMPORTANT
Earn 100
The correct order of intensity of colors of the compounds is:
(a)
(b)
(c)
(d)
25% studentsanswered this correctly

Important Questions on Coordination Compounds
HARD
JEE Main
IMPORTANT
Simplified absorption spectra of three complexes ((i) and (ii) and (iii)) of ion are provided below; their values are marked as and respectively. The correct match between the complexes and their values is:
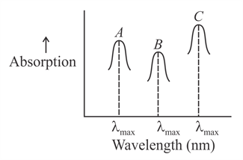
(i)
(ii)
(iii)
EASY
JEE Main
IMPORTANT
The one that is not expected to show isomerism is:
HARD
JEE Main
IMPORTANT
Consider that metal ion forms a complex with aqua ligands, and the spin only magnetic moment of the complex is . The geometry and the crystal field stabilization energy of the complex is :
MEDIUM
JEE Main
IMPORTANT
For octahedral and tetrahedral complexes, consider the following statements :
both the complexes can be high spin.
complex can very rarely be of low spin.
with strong field ligands, complexes can be low spin.
aqueous solution of is yellow in color.
The correct statements are :
MEDIUM
JEE Main
IMPORTANT
The oxidation states of iron atoms in compounds , and , respectively, are and . Then sum of and is ...........
MEDIUM
JEE Main
IMPORTANT
An octahedral complex with molecular composition M.5NH3.Cl.SO4 has two isomers, A and B. The solution of A gives a white precipitate with AgNO3 solution and the solution of B gives white precipitate with BaCl2 solution .The type of isomerism exhibited by the complex is :
EASY
JEE Main
IMPORTANT
Amongst the following, identify the species with an atom in oxidation state.
HARD
JEE Main
IMPORTANT
Nickel combines with a uninegative monodentate ligand to form a diamagnetic complex The hybridisation involved and the number of unpaired electrons present in the complex are respectively:
