MEDIUM
Earn 100
The correct stability order of carbocations is
(a)
(b)
(c)
(d)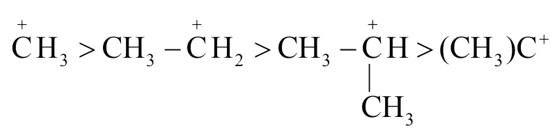
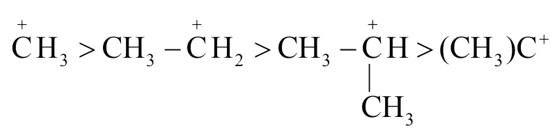
97.86% studentsanswered this correctly
Important Questions on Some Basic Principles of Organic Chemistry
EASY
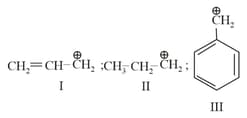
EASY
EASY
MEDIUM
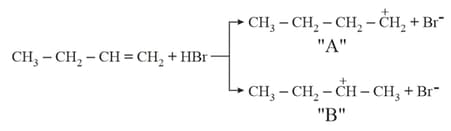
Choose the correct statement regarding the formation of carbocations A and B given :-
MEDIUM
MEDIUM
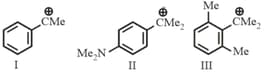
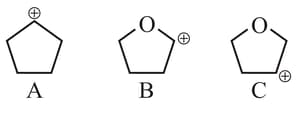
The stability order of the above carbocations is
MEDIUM
Arrange the following carbanions in order of their decreasing stability
(i)
(ii)
(iii)
HARD
Which of the following is most stable
MEDIUM
HARD
The major products and in the following reactions are
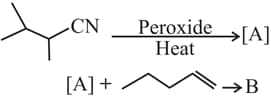
EASY
Arrange the following free radicals in order of decreasing stability:
MEDIUM
EASY
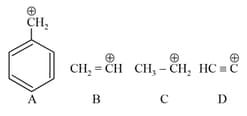
The correct order of stability of given carbocation is:
MEDIUM
Which of the following carbocations is most stable?
EASY
HARD
MEDIUM
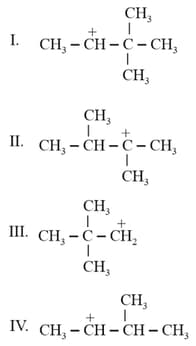
Arrange the following carbocations in decreasing order of stability.
EASY
I.
II.
III.
MEDIUM

follows the order
HARD

